40 phase labels in chemical equations
Mr. Jones's Science Class Understanding Chemical Formulas - Subscripts, Parentheses, Coefficients Totaling Atoms Using PSEC (PPT.) Analyzing Chemical Formulas Analyzing Chemical Equations Balancing Chemical Equations Balancing Chemical Equations (Online Tutorial) Balancing Equations Activity Find Someone Who... - Chemical Bonds and Reactions Carbon Monoxide Solutions Fluid Flow for Chemical Engineers - Free Pdf 4 Engineers Fluid Flow for Chemical Engineers. In preparing the second edition of this book, the authors have been concerned to maintain or expand those aspects of the subject that are specific to chemical and process engineering. Thus, the chapter on gas-liquid two-phase flow has been greatly extended to cover flow in the bubble regime as well as to ...
Factor-Label Method in Chemistry: Definition, Examples & Practice ... Factor-label, also known as dimensional analysis in some circles, describes a technique to convert one quantity - a length, mass, or anything else for that matter - to another quantity. The...

Phase labels in chemical equations
Electrochemcal Impedance Spectroscopy (EIS) Basics Phase angle represents the shift in phase when the input and output signals are overlapped in time. A complete EIS experiment consists of a sequence of sinusoidal potential signals centered around a potential setpoint. The amplitude of each sinusoidal signal remains constant, but the frequency of the input signal will vary. Action potential - Definition, Steps, Phases | Kenhub An action potential has three phases: depolarization, overshoot, repolarization. There are two more states of the membrane potential related to the action potential. The first one is hypopolarization which precedes the depolarization, while the second one is hyperpolarization, which follows the repolarization. An Open-Label Phase 1 Study of Ceralasertib in Japanese Patients With ... This is a Phase 1, open-label study designed to evaluate the safety, tolerability, pharmacokinetics, and anti-tumor activity of ceralasertib in Japanese patients with advanced solid malignancies. Cycle 0 duration is 4 days and each cycle from Cycle 1 has a duration of 28 days.
Phase labels in chemical equations. Chemical & Biological Engineering Research: Phase Diagrams The text explains how to use phase diagrams. The appendix includes the melting and boiling points of the elements, their allotropic transformation, and magnetic transition temperatures and crystal structures. Binary Alloy Phase Diagrams. T. Massalski, 3 vols., 2nd ed., 1990 Lockwood Library Science & Engineering Reference Collection TN 690 .B528 Cellular Respiration Equation, Types, Stages, Products & Diagrams Photosynthesis Equation The equation for photosynthesis of plant cells is: 6CO2 + 6 H2O + Sunlight → C6H12O6 + 6O2 ( 6 Carbon Dioxide + 6 Water + Sunlight → Glucose + 6 Oxygen ) Cellular Respiration Equation It is important to note that cellular respiration, in general, is not a single process but is, in fact, a set of metabolic reactions. Automating crystal-structure phase mapping by combining deep learning ... For example, in crystal-structure phase mapping, we use the k-sparsity constraint to implement the Gibbs rule (the number of phases than can coexist is, at most, three in a ternary chemical system ... Water Cycle - Process and its Various Stages - BYJUS There are many processes involved in the movement of water apart from the major steps given in the above water cycle diagram. Listed below are different stages of the water cycle. 1. Evaporation. The sun is the ultimate source of energy, and it powers most of the evaporation that occurs on earth. Evaporation generally happens when water ...
Photosynthesis- Definition, Equation, Steps, Process, Diagram The process of photosynthesis occurs in the thylakoids of chloroplasts. The process of cellular respiration occurs in mitochondria. The reactants of photosynthesis are light energy, carbon dioxide, and water. 6CO 2 + 6H 2 O → C 6 H 12 O 6 + 6O 2. The reactants of cellular respiration are glucose and oxygen. Examples of Physical Changes and Chemical Changes - ThoughtCo A chemical change results from a chemical reaction, while a physical change is when matter changes forms but not chemical identity. Examples of chemical changes are burning, cooking, rusting, and rotting. Examples of physical changes are boiling, melting, freezing, and shredding. Many physical changes are reversible, if sufficient energy is ... Photosynthesis - Wikipedia Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities.Some of this chemical energy is stored in carbohydrate molecules, such as sugars and starches, which are synthesized from carbon dioxide and water - hence the name photosynthesis, from the Greek ... CHE 105/110 - Introduction to Chemistry - Textbook The fundamental characteristic that all atoms of the same element share is the number of protons. All atoms of hydrogen have one and only one proton in the nucleus; all atoms of iron have 26 protons in the nucleus. This number of protons is so important to the identity of an atom that it is called the atomic number of the element.
Krebs cycle / Citric acid cycle / TCA Cycle with steps and diagram Krebs cycle Steps. Oxidative Decarboxylation of pyruvate to Acetyl CoA. Step 1: Condensation of acetyl CoA with oxaloacetate. Step 2: Isomerization of citrate into isocitrate. Step 3: Oxidative decarboxylations of isocitrate. Step 4: Oxidative decarboxylation of α-ketoglutarate. Step 5: Conversion of succinyl-CoA into succinate. 4.E: Chemical Reactions and Equations (Exercises) Write a chemical equation that represents (NH 4) 3 PO 4 (s) dissociating in water. Write a chemical equation that represents Fe (C 2 H 3 O 2) 3 (s) dissociating in water. Write the complete ionic equation for the reaction of FeCl 2 (aq) and AgNO 3 (aq). You may have to consult the solubility rules. Chemistry Lab Resources (for CHM 1XX and 2XX Labs) - Purdue University Given enough information, one can use stoichiometry to calculate masses, moles, and percents within a chemical equation. Moles and Avogadro A mole simply represents Avogadro's number (6.022 x 10 23) of molecules. A mole is similar to a term like a dozen. If you have a dozen carrots, you have twelve of them. 6.7: ODE and Excel Model of an Adiabatic PFR The equation to describe conversion as a function of volume, as derived from a simple mole balance, is shown below in Equation 6.7.1. (6.7.1) d X d V = − r A F A O X= conversion V= volume rA = reaction rate of A FAo = initial moles of A 4. Rate Law The rate law is independent of reactor type.
Glycolysis : All Steps with Diagram, Enzymes, Products, Energy Yield ... Phases of Glycolysis There are two main phases of glycolysis. The energy-requiring phase (Preparatory phase) The energy-releasing phase. (Payoff phase) Glucose-requiring phase (Preparatory Phase) This phase is also called the glucose activation phase.
5 Types of Bonds in Chemistry with Examples and Diagrams - Study Read Covalent bonds are of two types as. Nonpolar covalent bond and. polar covalent bond. A non-polar bond is one where the two atoms share the atoms equally; it is known as a non-polar/pure covalent bond. This occurs when the atoms participating in the bond are the same. This is the case in hydrogen gas ( H2), oxygen gas ( O2), nitrogen gas (N2), etc.
Guidance for Selecting Input Parameters in Modeling the ... - US EPA The primary sources of input fate parameters that are used in these models include pesticide product chemistry and labeling information as well as sorption coefficients, half-lives, and rate constants from acceptable or supplemental environmental fate studies conducted or sponsored by pesticide manufacturers.
9.4: PID tuning via Frequency Responses with Bode Plots To find the phase shift, the periods of the input and output sine curves need to be found. Recall that the period, P, is the length of time from one peak to the next. P = 1 f = 2π ω with f is the frequency and ω is the angular frequency (rad/sec) The phase shift is then found by ϕ = − 2π(ΔP P)
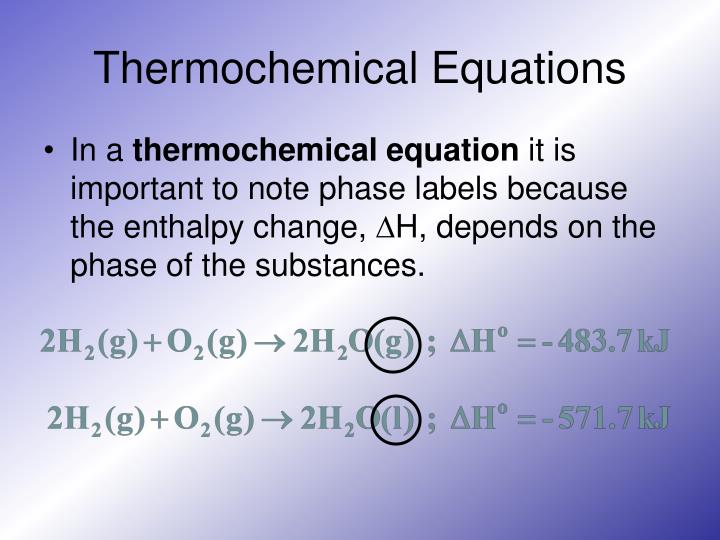
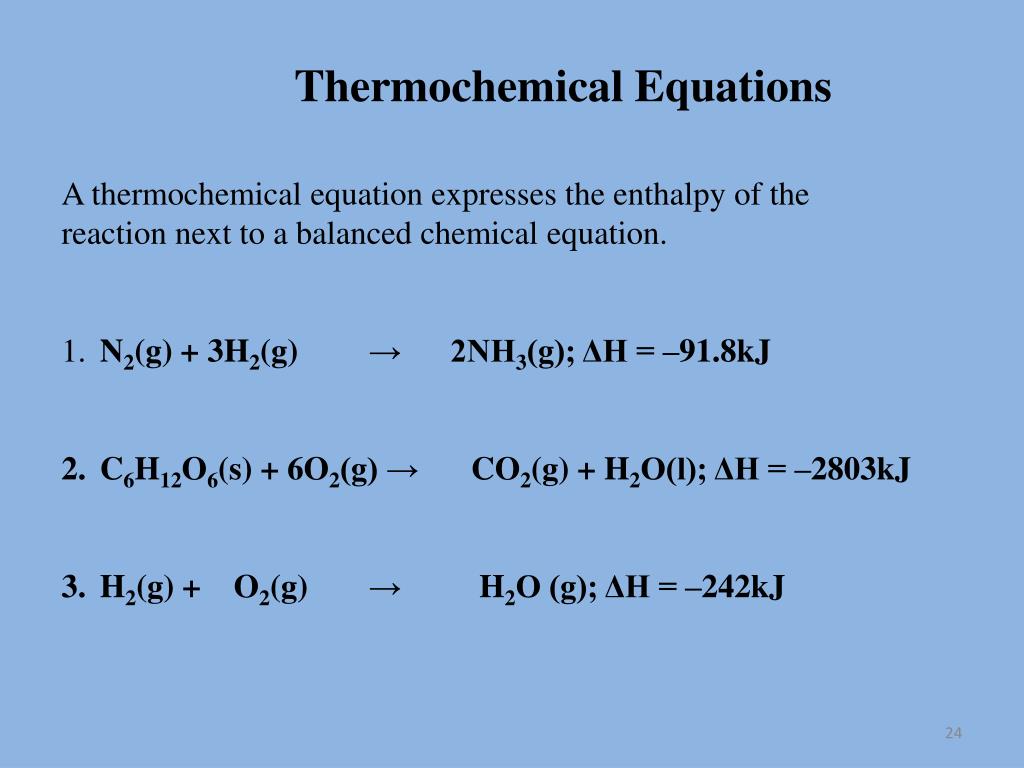
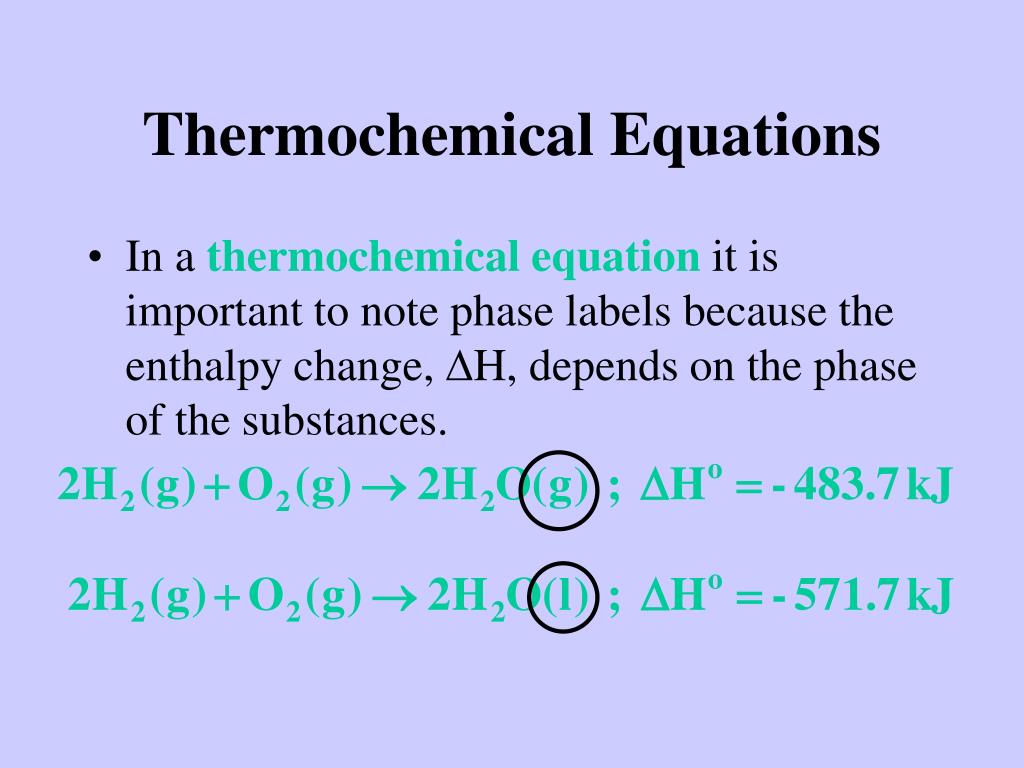
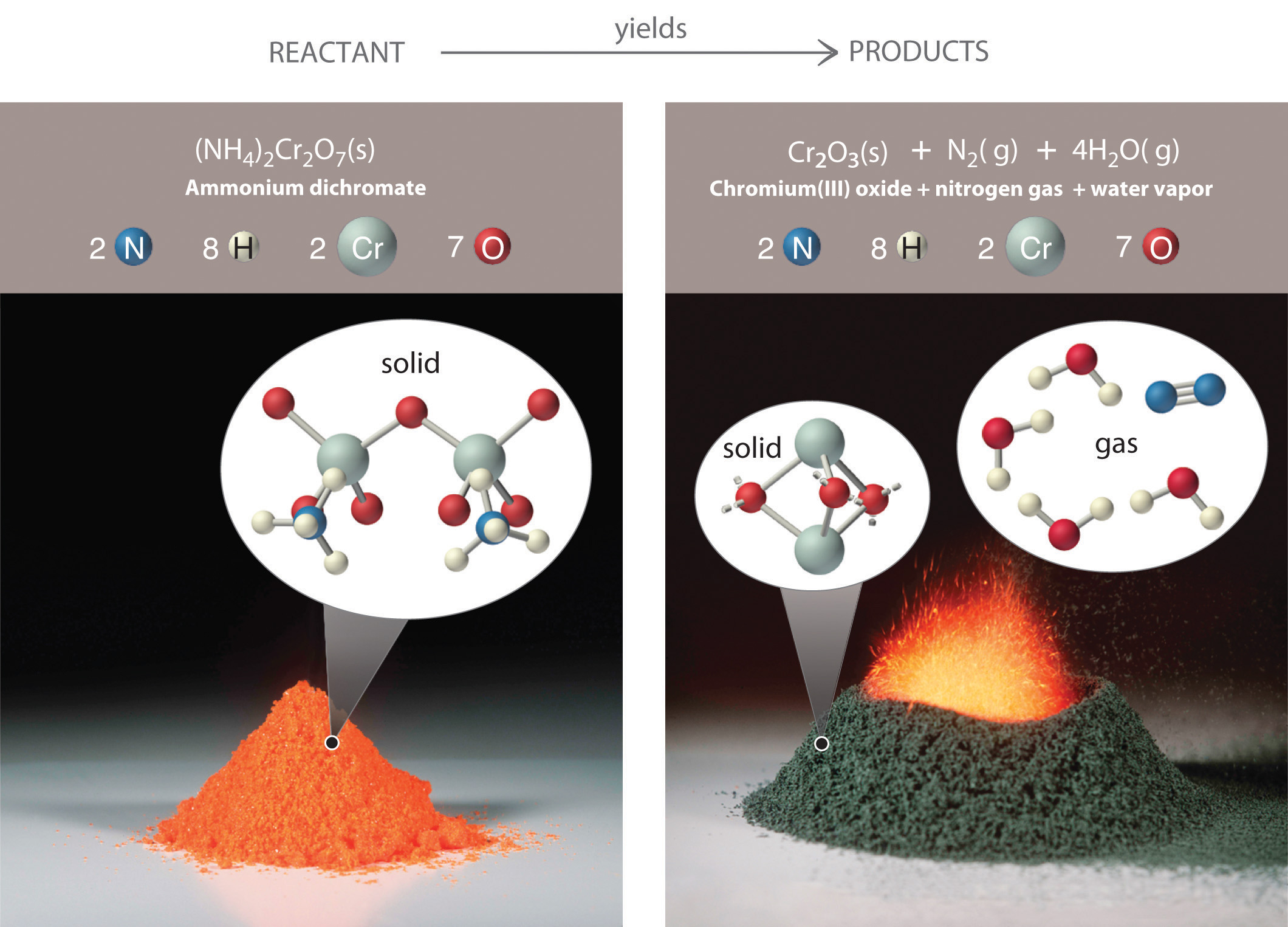
4.2: The Chemical Equation - Chemistry LibreTexts Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2 NaHCO 3 ( s) → 200 ∘ C Na 2 CO 3 ( s) + CO 2 ( g) + H 2 O ( l) Key Takeaways
Symmetry in Crystallography Notes - University of Oklahoma As in the proper rotation operations, only 1 ( i = S2 ), m = 2 (σ = S1 ), 3 ( S6 ), 4 ( S4 ), and 6 ( S3) improper rotations are commonly observed in crystals. These axes are pronounced as 3 bar in the United States and bar 3 in many European countries. Thus 3 in H-M is equivalent to S6 in Schönflies.
Basic Principles of HPLC, MS & LC-MS | Chemyx Inc When a sample is injected, it is adsorbed on the stationary phase, and the solvent passes through the column to separate the compounds one by one, based on their relative affinity to the packing materials and the solvent. The component with the most affinity to the stationary phase is the last to separate.
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways
Glycolysis Explained in 10 Easy Steps - Microbiology Info.com In this step, two main events take place: 1) glyceraldehyde-3-phosphate is oxidized by the coenzyme nicotinamide adenine dinucleotide (NAD); 2) the molecule is phosphorylated by the addition of a free phosphate group. The enzyme that catalyzes this reaction is glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
CBSE 10th Science Exam 2022: Most Important Chemical Reactions ... Check important chemical reactions and diagrams based on which questions are expected to be asked in the CBSE Class 10 Science Term 2 Exam 2022. In CBSE Class 10 Board Exams, questions based on ...
An Open-Label Phase 1 Study of Ceralasertib in Japanese Patients With ... This is a Phase 1, open-label study designed to evaluate the safety, tolerability, pharmacokinetics, and anti-tumor activity of ceralasertib in Japanese patients with advanced solid malignancies. Cycle 0 duration is 4 days and each cycle from Cycle 1 has a duration of 28 days.
Action potential - Definition, Steps, Phases | Kenhub An action potential has three phases: depolarization, overshoot, repolarization. There are two more states of the membrane potential related to the action potential. The first one is hypopolarization which precedes the depolarization, while the second one is hyperpolarization, which follows the repolarization.
Electrochemcal Impedance Spectroscopy (EIS) Basics Phase angle represents the shift in phase when the input and output signals are overlapped in time. A complete EIS experiment consists of a sequence of sinusoidal potential signals centered around a potential setpoint. The amplitude of each sinusoidal signal remains constant, but the frequency of the input signal will vary.
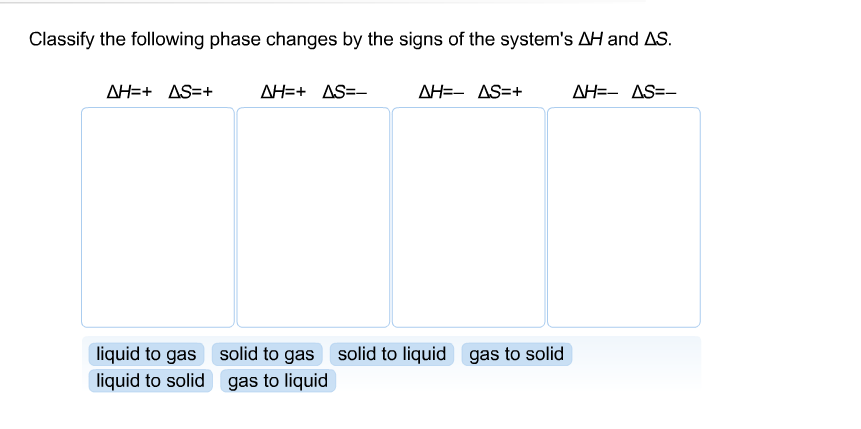
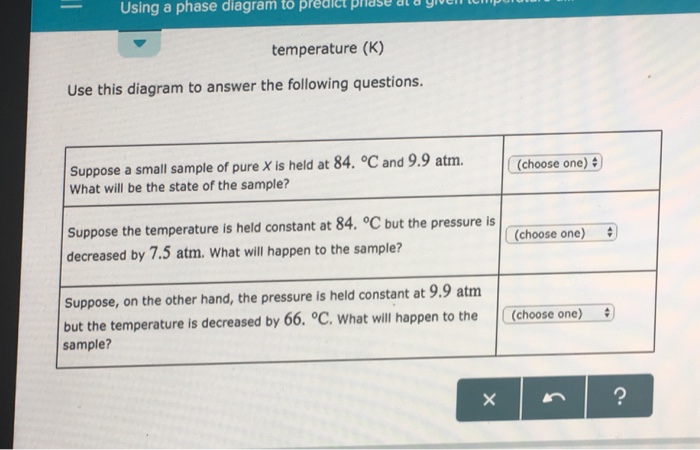







Post a Comment for "40 phase labels in chemical equations"